There is a correlation between diet and health and lifespan of genetically diverse mice
by admin
An Analysis of Longitudinal Disease Effects on Lifetime using Genetic Mapping for Continuous-Valued Traits and Genomic Variables
The findings could lead to a different perspective on how scientists think about studies of diet. In one of the most comprehensive clinical trials of a low-calorie diet in healthy, non-obese individuals, researchers found5 that the intervention helped to dial down metabolic rates — a short-term effect thought to signal longer-term benefits for lifespan.
To outside investigators, the results drive home the intricate and individualized nature of the body’s reaction to caloric restriction. James Nelson is a biogerontologist at the University of Texas Health Science Center in San Antonio.
The study, funded by the anti-wrinkle focused medical research company, was published today in the journal, Nature.
Daniel Belsky cautions against overpolating from mice to humans. The study added to the growing knowledge that lifespan and healthspan are not the same thing.
Genetic mapping analysis of all continuous-valued traits including lifespan was performed using the R package qtl263. Additive covariates for the genome-scan models were diet and body weight variables as indicated in Supplementary Table 5. Founder haplotype effects were estimated treating 8-state genotypes as random effects. Trait heritability was estimated using the R/qtl2 function est.herit() to fit a linear mixed model including an additive kinship matrix. Confidence intervals were obtained by parametric bootstrap. The significance thresholds for QTL mapping were calculated from the 1,000 permutations of the trait and comune data.
We obtained a covariance decomposition by recomputing a sparse low-rank network on a reduced representation of features. The relative importance of different effects of DR on lifespan can be determined by using this reduced representation graphical model. The absolute values of path scores were used to rank the relative importance of each path and normalized to sum to 1 to estimate the fraction of the covariance between DR and lifespan explained. The position of the edges were determined by the max-flow through the path network. We define the path network as the graph formed by taking the partial correlation network but reweighting edges to be the sum of all (absolute) path scores of paths that contain that edge.
where T is trait, D is dietary assignment, BW is body weight at the date closest to T’s collection date, PLL is the proportion of life lived as of T collection date and s() is the smoothing parameter. Each mouse had multiple datapoints across the T collection date. This clustering was accounted for with a random intercept for ID, specified as (1|ID) above. We performed hypothesis tests related to the GAMM fits to explore trait sensitivity to body weight (model 1 (M1) versus M2), PLL, (M1 versus M3), diet (M1 versus M4) and diet-by-trait interaction (M1 versus M5). Using the models specified above and a conservative false-discovery rate (FDR < 0.01, one-step Benjamini–Hochberg method), we therefore identified traits that responded additively to body weight, traits that responded additively to diet, traits that responded additively to PLL (scaled age) and traits that responded interactively to diet and PLL. Traits were categorized as health, metabolism, haematology or immune. For each trait category, bar plots were generated to show the number of traits with significant (FDR < 0.01) associations with body weight, diet, PLL and diet × PLL interactions. We took the sameanalysis with age in months as before. We applied FDR adjustment to each test across traits and timepoints (Benjamini–Hochberg FDR method).
where BW6 is the last preintervention body weight, and BWtest is the body weight at time of testing. Body weight terms were not included for body composition and change-in-body weight traits. All continuous variables were rank normal scores (RZ) transformed before model fitting. We performed likelihood ratio tests for the diet and body weight adjusted association (M2 versus M1) and for the diet × trait interaction (M3 versus M2). The FDR adjustment was applied to the tests based on trait and timepoints. The results were determined using the same methods as before: traits were categorized and significant results were calculated.
The activity, feeding, and respiration of the individually housed mice were tracked after they were housed in a cage for 7 days. Feeding protocols for dietary intervention were maintained. Metabolic cage data were used to assess metabolism, energy expenditure and activity of mice in Y1, Y2 and Y3. The data has been cleaned out to remove outliers and instrument failure and summarized as a cumulative or median over 5 minutes. The mean and s.d. were plotted as a mean 2 s.e.m at 4 and 1 h intervals. Across timepoints. Animal-level summaries were computed as the average across 7 days of the daily (24 h), light phase (12 h) or dark phase (12 h) median values. Moreover, we computed ‘change’ traits (for example, delta respiratory quotient and delta energy expenditure) as the difference between the 5th to 95th percentiles of all 1 h summaries across the entire 7 day run.
There were a number of pre-defined clinical symptomology for mice that were evaluated by research staff. If mice met the criteria for observation in any of these categories, veterinary staff were contacted. If the clinical team determined that an animal was irretrievably hypothermic and unresponsive and could not eat or drink, they would pre-emptively put it down so it wouldn’t suffer. The survival curves show both the euthanized and dead mice as deaths. Of the 13 events recorded, 13 had mice euthanized due to unrelated injuries to imminent death.
Free-wheel-running data were collected at around 44, 96 and 144 weeks of age. The mice were housed in a special cage with a low profile running wheel and a wireless transmitter, for a minimum of 36 h. The food hopper was removed to allow for seamless movement of the wheel, and food was placed onto the cage floor. The 15.5-cm-diameter plastic wheel sits at an angle on an electronic base, which tracks the revolutions. Continuous monitoring of data can be accomplished with the battery- powered base, which allows for 30 s wireless transmission to a local computer.
There was a piece of blotting paper cut to standard duplex cage dimensions. Paper was taped to the bottom of a cage after shavings were removed. Food was provided during this test; however, water was removed to prevent possible leaking onto the blotting paper. Mice were individually housed in a prepared cage for 4 h. At the end of the trial, the mice were returned to their original housing units, and papers were removed and dried for 2–4 h, before being individually bagged. The papers were sent to the medical center to be scanned and quantify the void spots.
The Ugo-Basile rotating rod allowed for up to five mice to be tested at the same time. A platform below each lane holds a trip plate that records the latency for each mouse to fall. At the beginning of the session, mice were placed onto the rod, which began rotating at 4 rpm, slowly increasing to a maximum of 40 rpm, over 300 s. Mice were given three consecutive trials. We reported the mean delay, slope of latencies, number of trials with no falls, and number of trials with immediate falls. In case a mouse did not cooperate with the test, trials were recorded as missing.
Startle response was measured in rodents using automated startle chambers, in which a mouse was placed in a clear, acrylic tube attached to a highly sensitive platform that is calibrated to track their startle reflex while being exposed to a series of stimuli at varying decibels and times. A series of randomized, computer-generated stimuli ranging in volume from 70 to 120 decibels at 40ms in duration and an interval of 9–22 s are what the mice were exposed to after initial exposure to white noise. The test runs for approximately 30 min.
Flow cytometry of outbred DO mice using pulsed Doppler ultrasound with LifeScan and OneTouch Ultra2 : Application to peripheral blood
Ultrasonography was accomplished using the Vevo 770/2100 system with 30 and 40 MHz probes. Blood flow rates and volumes can be measured using echocardiography, which uses a pulsed Doppler sonography probe.
At the flow cytometry blood collections at 16, 62 and 114 weeks, mice were fasted for 4 h and glucose was measured using the OneTouch Ultra2 glucose meter from LifeScan along with OneTouch Ultra test strips. The non-fasted sugar was measured using a meter at each of the three blood collections.
Owing to the outbred nature of these mice, flow cytometry markers were limited, and T cell subsets were generally assigned as naive and non-naive by the presence of CD62L and CD44 (immune cell subtype designations are shown in Supplementary Table 7). NKG2D-positive cells were enumerated and may represent memory T cells that accumulate after immune responses60. Owing to limitations in flow cytometry markers that identify NK cells and their subsets in the mouse strains contributing to the outbred DO mouse line, NK cells were defined as non-T non-B lymphocytes expressing NKG2D. Within this population, CD11c and CD11b were used to generally define maturation subsets. CD11b expression marks more mature NK cells and CD11c is reduced on the least-mature NK subset61.
Flow cytometry determined the frequencies of major immune cell subsets with the help of peripheral blood samples. Before the start of intervention at the beginning of 5 months, it was recommended to have the analysis done. The timepoints correspond to 11 and 19 months of intervention. Red blood cells in PBL samples were lysed and the samples were washed in FACS buffer (Mitenyi, 130-091-222). Cells were resuspended in 25 μl FACS buffer with 0.5% BSA (Miltenyi, 130-091-222 with 130-091-376). Antibodies including Fc block (2.42, Leinco Technologies) were added and incubated for 30 min at 4 °C. Labelled cells were washed and DAPI was added before analysis on the LSRII (BD Bioscience) system. The antibody cocktail contained CD11c FITC, N418 (35-0114-U100, Tonbo Biosciences, 1:100); NKG2D (CD314) PE, CX5 (558403, BD Biosciences, 1:80); CD3e PE-CF594, 145-2C11 (562286, BD Biosciences, 1:40); CD19 BB700, 1D3 (566411, BD Biosciences, 1:40); CD62L PE-Cy7, MEL-14, (60-0621-U100, Tonbo Biosciences, 1:100); CD25 APC, PC61 (102012, BioLegend, 1:80); CD44 APC-Cy7, IM7 (25-0441-U100, Tonbo Biosciences, 1:40); Ly6G BV421, 1A8, (562737, BD Biosciences, 1:80); CD4 BV570, RM4-5 (100542, BioLegend, 1:40); CD11b BV650, M1/70 (563402, BD Biosciences, 1:160); CD45R/B220 BUV496 (RA3-6B2, 564662, BD Biosciences, 1:20); Fc Block, 2.4G2 (C247, Leinco Technologies, 1:100).
Source: Dietary restriction impacts health and lifespan of genetically diverse mice
Double X-ray absorptiometry analysis of DO mice using the LUNAR PIXImus II densitometer
We performed dual X-ray absorptiometry analysis using the LUNAR PIXImus II densitometer to collect bone density and body composition (including fat and non-fat lean tissue). Mice were anaesthetized and individually placed onto a disposable plastic tray that was then placed onto the exposure platform of the PIXImus. The process to acquire a single scan lasts approximately 4 min. The age at which the measurement was taken was around 44 years old.
We performed three cycles of health assessments of mice at early, middle and late life. These assessments included a 7-day metabolic cage run at around 16, 62 and 114 weeks of age; blood collection for flow cytometry analysis at 24, 71 and 122 weeks; rotarod, body composition, echocardiogram, acoustic startle, bladder function, free wheel running and a blood collection for CBC analysis at around 44, 96 and 144 weeks of age. Furthermore, body weights were recorded weekly and manual frailty and grip strength assessments were carried out at 6 month intervals. All assays were conducted at the Jackson Laboratory according to standard operating procedures.
Over 100,000 values were collected for 937 mice as they were weighed weekly throughout their lives. Body weights were analysed after local polynomial regression fitting within mouse (that is, loess smoothing).
To obtain an accurate estimate of food intake and changes in body weight as a result of weekly fast cycles, we set up an independent cohort of female DO mice. The DR protocols of the mice were the same as in the main study. When the mice were younger than 30 days of age, the food was weighed daily. The daily and weekly averages were presented for the food intake data normalized to units of g per mouse. Body composition was determined at 43 and 45 weeks of age using non-imaging nuclear magnetic resonance (NMR) using the Echo MRI instrument, a NMR device with a 5-gauss magnet that is adapted to small-animal studies. NMR data were used to detect changes in body weight and composition before and after fasting. Values of body weight, lean mass, fat mass and adiposity (100% × fat mass/total mass) pre-fasting were co-plotted with the difference between before and after fasting (Friday to Monday for AL and CR; Tuesday to Thursday for 1D IF; Tuesday to Friday for 2D IF).
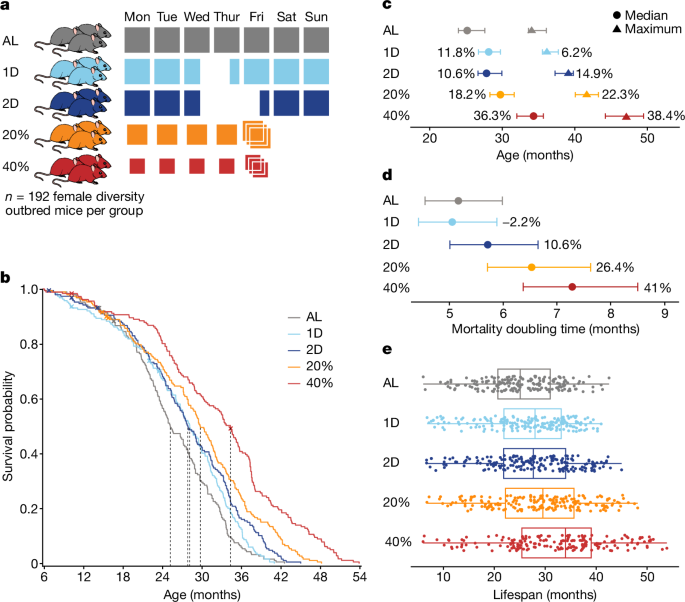
DR was implemented by controlling the timing and amount of food provided to mice. Feeding schedules for DR were started at 6 months old. All mice were fed a standard mouse chow diet (5K0G, LabDiet). The AL feeding group was provided with unlimited access to food and water. The IF mice were provided unlimited access to food and water. On Wednesday of each week at 15:00, IF mice were placed into clean cages and food was withheld for the next 24 or 48 h for the 1D and 2D groups, respectively. CR mice were provided with unlimited access to water and measured amounts of food daily at around 15:00, 2.75 g per mouse per day for 20% CR and 2.06 g per mouse per day for 40% CR. Historical feeding data from DO mice gave us an estimated ALA consumption of 3.43 g per mouse per day. For the 40% CR protocol, a gradual reduction in food intake was implemented: the mice were first subjected to 20% CR for 2 weeks, then to 30% CR for an additional 2 weeks, before transitioning to the full 40% CR. The first 2 weeks are when the mice are acclimatized to the 1D IF regimen. There were up to eight mice in a pen. The standard practice for CR studies is co-housing, where food is placed in the bottom of the cage, allowing individual mice to grab a pellet and put it in their mouth. The 20% CR mice were provided with a 3 day ration of food on Friday, followed by a period of famine, similar to the intermittent hunger periods seen in the IF, for two days. The feeding time is close to the normal time of day for mice and starts before the beginning of the dark cycle. This timing has been shown to maximize lifespan extension in mice subjected to 30% CR8. The feast–famine cycle can be used in studies of CR 57, 58,59, but there is not a direct assessment of the health impacts.
A 7-day metabolic cage run at around 16, 62 and 114 weeks of age was used to monitor activity, feeding, and respiration of individually housed mice. These assessments included a seven-day metabolic cage run at around 16, 62 and 114 weeks of age; flow analysis at 24, 71 and 122 weeks; T cell subsets were mostly assigned as naive and non-naive by the presence of CD62L and CD44.