The FDA approved the first treatment for a human illness
by admin
The first NPR patient to receive CRISPR treatment in the U.S. received by a Forest, Miss., sickle cell patient
The approval of the CRISPR gene-editing treatment was also welcomed by Victoria Gray, a Forest, Miss., sickle cell patient who was the first person to receive it in the U.S. NPR has had exclusive access to chronicle her experience since she was treated in 2019.
Some patients can be cured by bone marrow transplants, but most can’t find a suitable donor. About 20,000 patients in the U.S. have the severe form of the disease the CRISPR treatment would initially be used to treat.
Fetal haemoglobin is produced when the edited cells produce a form of red blood cells. The brand name of the therapy is designed to alleviate symptoms for a lifetime, and not be a cure for the disease.
Data presented to the FDA showed that the treatment resolved the pain crises for 29 subjects who were at least 18 months old. Similarly, similar results have been produced by the treatment for patients suffering from a related condition.
Prices for Lyfgia and Other Rare Gene-Editing Treatments: Implications for Brain and Spinal Mechanics
They are both very expensive. The wholesale price is believed to be over $2 million. Bluebird set the wholesale price of Lyfgenia at $3.1 million.
Aside from the price for the treatments, another concern is the procedures are long, difficult and complex, requiring multiple trips to a hospital for testing, a grueling and potentially dangerous bone marrow transplant, and lengthy hospitalization. Those factors may make it hard for people in the US who need the treatment the most to get it.
Gene-editing, which allows scientists to manipulate the basic building blocks of life more easily than ever before, is being studied as a treatment for illnesses ranging from rare genetic disorders like muscular dystrophy to common ailments like cancer, heart disease, diabetes, AIDS and Alzheimer’s.
A New Beginning for People with Sickle Cell Disease: First Gene Editing Therapies for Human Illness (FDA approves first gene editing treatments for human illness)
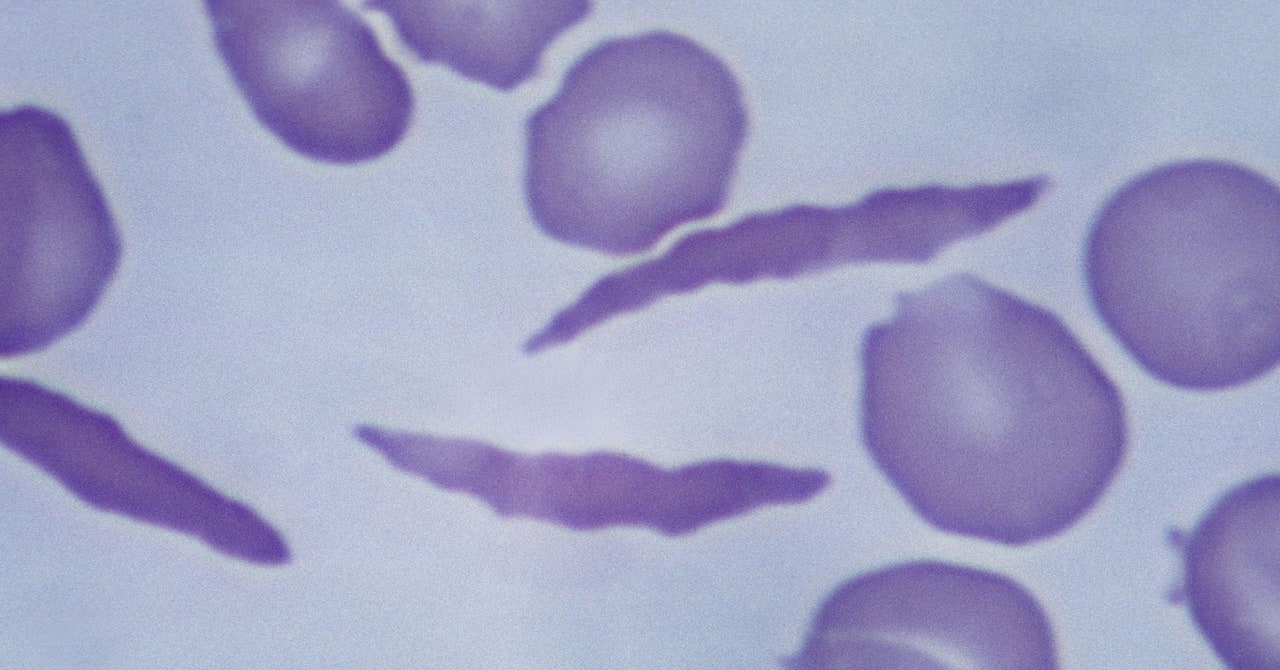
Sickle cell disease is a hereditary condition in which a person’s red blood cells are hard and crescent-shaped instead of flexible and round. These sickled cells block the flow of blood, causing big bouts of pain. Over time, the disease can cause organ damage. Sickled cells also die off early, leaving a shortage of healthy red blood cells. A low red blood cell count means the body can’t get enough oxygen, a condition called anemia. This causes fatigue, as well as respiratory problems. The life expectancy for people with the disease is two decades less than the rest of the US population.
Gray was forced to rush to the hospital countless times for blood and drugs as a patient of the Sickle cell disease. She was stuck with no choice but to care for herself or her children.
Since the treatment, Gray’s has been much more energetic and able to start working full time selling cosmetics at Walmart and spend more time with her four children, who are now teenagers.
“Since I received the CRISPR treatment, I’ve had a new beginning. “I don’t have to worry about dying or leaving my children behind without a mom,” Gray says. “My life is not limited anymore.” I’m full of energy. I do not have pain. It is a real transformation.
Creary has a disease herself and is a professor at the University of Michigan School of Public Health. “I am excited about the promise that this technology has for those living with sickle cell disease. As technology comes to market, it’s going to be interesting to see how profit is going to surpass social justice.
Many of the countries where most sickle cells patients live don’t have enough sophisticated medical centers to provide the complicated treatment. Even in the U.S., the treatment may not be widely available, making it difficult to access.
Source: FDA approves first gene-editing treatment for human illness
CRISPR-Based Treatments of Rare Liver Diseases and Family-Hypercholesterolemia at the National Institutes of Health
Another concern is whether sufficient research had been done to spot “off-target” effects of the treatment — unintended editing errors that missed their mark in the DNA and that could potentially cause long-term health problems.
The companies will follow each patient for 15 years to see how long the benefits last if the treatment prolongs lifespan.
CRISPR based treatments have also shown promise for treated a rare liver condition known as amyloidosis, as well as an inherited form of high cholesterol known as familial hypercholesterolemia.
The National Human Genome Research Institute is at the National Institutes of Health, and Vence Bonham is acting deputy director. It could change the lives of individuals and reduce the burden of pain episodes.
Victoria Gray, a sickle cell patient who was the first person to receive CRISPR gene-editing treatment in the US, said she is “excited about the promise that this technology has for those living with sickle cell disease.” “Many of the countries where most sickle cell patients live don’t have enough sophisticated medical centres to provide the complicated treatment,” she added.