Azaarenes are carbon-to-nitrogen transmutations
by admin
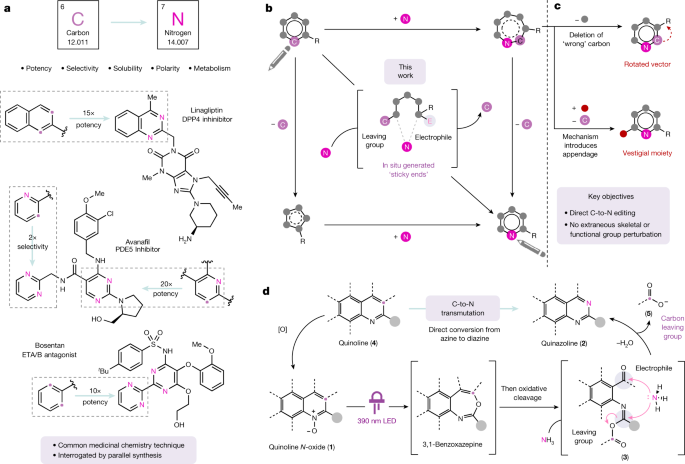
The necessary nitrogen atom in chemical biology and drug discovery: from bosentan to macitentan (Opsumit®)
Pennington, et al., harness the nitrogen atom in chemical biology and drug discovery. Med. Chem. Res. https://doi.org/10.1007/s00044-023-03073-3 (2023).
There are surprising effects of C–H methylation in drug discovery. Angew. Chem. Int. Ed. 52, 12258-1296.
Pennington, L. D. & Moustakas, D. T. The necessary nitrogen atom: a versatile high-impact design element for multiparameter optimization. J. Med. is a medical journal. Chem. 60, 3552–3579 (2017).
Analysis of the structural diversity, substitution patterns, and Frequency of Nitrogen Heterocycles among U.S. FDA approved pharmaceuticals. J. Med. Chem. 57, 10257–10274 (2014).
Boss, C., Bolli, M. H. & Gatfield, J. From bosentan (Tracleer®) to macitentan (Opsumit®): the medicinal chemistry perspective. Bioorg. Med. Chem. The Lett. 26 is published in the summer.
Eckhardt, M., Klein, T., Nar, H. & Thiemann, S. in Successful Drug Discovery (eds Fischer, J. & Rotella, D. P.) 129–156 (Wiley, 2015); https://doi.org/10.1002/9783527678433.ch7
Kelley, B. T., Walters, J. C. & Wengryniuk, S. E. Access to diverse oxygen heterocycles via oxidative rearrangement of benzylic tertiary alcohols. Org. Lett. 18 1896- 99.
Siddiqi, Z., Wertjes, W. C. & Sarlah, D. Chemical equivalent of arene monooxygenases: dearomative synthesis of arene oxides and oxepines. J. Am. Chem. Soc. 142, 10125–10131 (2020).
Polarization induced skeleton editing through nitrogen deletion in alkyl ethers. Nature 229, 222,222 ( 2016)
Kennedy, Dherange, B. D., Berger, K.J., and M. D. Skeletal editing through nitrogen deletion. Nature 229,222,222 ( 2016).
Lyu, H., Kevlishvili, I., Yu, X., Liu, P. & Dong, G. Boron insertion into alkyl ether bonds via zinc/nickel tandem catalysis. Science 372, 175–182 (2021).
The first ring-opening and second ring-closing reaction for converting para-substituted pyridines into meta-substituted anilines was done by Morofuji. It is an organization with the singular purpose of keeping tabs on all things Organization. Lett. 23-26, 6136–6130.
Late-stage diversification of indole skeletons through nitrogen atom insertion. Science 377, 1104–1109 (2022).
Spence, G. G., Taylor, E. C. & Buchardt, O. Photochemical reactions of azoxy compounds, nitrones, and aromatic amine N-oxides. Chem. Rev. 70, 231–265 (1970).
A., Tsukamoto, T., and G, cut and sew transformation into metal-catalyzed carbon bond activation. ACS Catal. 7, 1340–1360 (2017).
Boyle, B. T., Levy, J. N., de Lescure, L., Paton, R. S. & McNally, A. Halogenation of the 3-position of pyridines through Zincke imine intermediates. Science 378 and 773 were published in a single year.
Source: Carbon-to-nitrogen single-atom transmutation of azaarenes
DNA cutter: ozonolysis of the acetylated polyelectrolytes AMG 232 and preparation of the Bisulfite Adduct of 2-hydroxyindan-2-carboxaldehy
A., Wilson, G.G., and Murray, N. E., present the highlights of the DNA cutter. A primer on the subject of the Nucleic Acids Res. 42.
Pyridine is an organocatalyst that allows the oxidation of alkenes. It’s listed on the Organization’s website Lett. 14, 2224– 2235 of 2012
The quinoline N-oxides with -oxocarboxylic acids can be acylated by a regioselective C–H bond. Org. Lett. 18, 3722–3725 (2016).
Shieh, P., Hill, M. R., Zhang, W., Kristufek, S. L. & Johnson, J. A. Clip chemistry: diverse (bio)(macro)molecular and material function through breaking covalent bonds. Chem. Rev. 121, 7059–7121 (2021).
Cochran, B. M. et al. Development of a commercial process to prepare AMG 232 using a green ozonolysis–Pinnick tandem transformation. The J.org. Chem. 84, 4763–4779 were published in 2019.
Ragan, J. A. et al. Safe execution of a large-scale ozonolysis: preparation of the bisulfite adduct of 2-hydroxyindan-2-carboxaldehyde and its utility in a reductive amination. Org. Process Res. Dev. 7, 155–160 (2003).
Source: Carbon-to-nitrogen single-atom transmutation of azaarenes
Me-Talnetant and Osanetant interactions in human tachykinin neurokinin 3-receptor transmembrane domains
Malherbe, P. Me-Talnetant and Osanetant interact within overlapping but not identical binding pockets in the human tachykinin neurokinin 3 receptor transmembrane domains. Mol. Pharmacol. 73, 1736–1750 (2008).
Dexter, D. L. et al. Activity of a novel 4-quinolinecarboxylic acid, NSC 368390 [6-fluoro-2-(2′-fluoro-1,1′-biphenyl-4-yl)-3-methyl-4-quinolinecarboxylic acid sodium salt], against experimental tumors. The Cancer Res. 45 was published in 1985.
Wojciechowski, B. J., Chiang, C. Y. & Kuczkowski, R. L. Ozonolysis of 1,1-dimethoxyethene, 1,2-dimethoxyethene and vinyl acetate. J. Org. Chem. 55, 1120–1122 (1990).
Gollnick, K. and Koegler, S., analyzed thermal transformations of oxazole endoperoxides. Tetrahedron Lett. 29, 1007–1010 (1988).
The interpretation and realization of catalytic activity with formamide-catalyzed nucleophilic substitution. The year 2020 is marked by the publication of the ACS Catal. 10.
The nitrogen atom plays a critical role in drug discovery by mediating oxygen-to-oxygen transitions. An analysis of the structural diversity, substitution patterns, and Frequency of Nitrogen Heterocycles among US FDA approved pharmaceuticals revealed that nitrogen atom insertion increased oxygen-to-oxygen transition from bosentan to Macitentan. The nitrogen atom is also required for diversification of indole skeletons through nitrogen atom insertion.