If you test these techniques outside of the lab, you can see that chemicals can be destroyed with clever chemistry
by admin
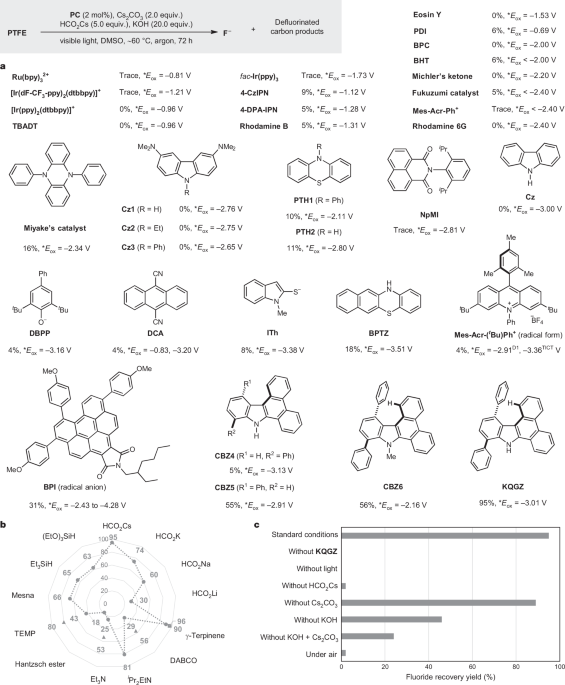
Photocatalytic reductive cleavage of carbon-fluorine bond by CBZ6: A study at Nagai, Y., Smith, R. L.Jr. and Arai, K
Nagai, Y., Smith, R. L.Jr., Inomata, H. & Arai, K. Direct observation of polyvinylchloride degradation in water at temperatures up to 500°C and at pressures up to 700 MPa. J. Act. Polym. Sci. 106, 1075–1086 (2007).
The C–F functionalization of un active trifluoromethylarenes is improved by this work. J. Am. There is a drug called Chem. 141, 13203 and 13211 were published in 2019.
The carbon–fluorine (C–F) bond is one of the strongest in organic chemistry, requiring huge amounts of energy to break down, at huge expense. There are two low-energy ways to get rid of the C–F bond.
Yabuta, T., Hayashi, M. & Matsubara, R. Photocatalytic reductive C–O bond cleavage of alkyl aryl ethers by using carbazole catalysts with cesium carbonate. J. organization. Chem. 86, 2545– 2555 will be published in 2021.
Aeductive cleavage of C–X or N–S bonds by CBZ6 was studied. It is the company’s name Lett. 25 has a date of 2023
Z. F., Xiao, and others. There was a way to cyclization isoxazolines and alkenes with diverging access to pyrrolidines, pyrroles and carbazoles. It is called the Organization. Lett. 18, 5672–5675 (2016).
Chemistry of Fluoropolymers: End of life assessment and chemical formula for Chem. Int’l 135, 22403-22412 (20023)
Halder, S., Mandal, S. and Kundu, A. are all part of a team that discovered the super-reducing behavior of a drug. J. Am. The chemical formula for Chem. Int’l 135, 22403–22412 (20023).
The article was written by K, and it is titled “Zheng, K.” A phenolate anion under visible light makes it possible to convert aryl halides and TEMPOH into omers. Chem. The journal, Sci. 11, has a special article about the 2020 edition.
Aeductive electrophotocatalysis is the merging of electricity and light to achieve extreme reduction potential. J. Am. Chem. 142, 2089–1092 was reported in the year 2020.
Wu, Y., Kim, D. & Teets, T. S. Photophysical properties and redox potentials of photosensitizers for organic photoredox transformations. Synlett 33 was recorded in the year 2022.
A room temperature deflorinating of poly(tetrafluoroethylene) by a magnesium reagent was done. J. Am. The substance that is Chem. There is an article in a society called 10486–10490 (2023).
Costello, C. A. & McCarthy, T. J. Surface-selective introduction of specific functionalities onto poly(tetrafluoroethylene). Macromolecules 20, 2819–2828 (1987).
Ellis, D.A., Mabury, S. A., Martin, J.W., and Muir, D. C.G. have discovered a potential source of halogenated organic acids in the environment. Nature 412, 321–324 (2001).
Améduri and Hori discussed what recent developments, challenges and future trends are on end of life assessment of fluoropolymers. That is Chem. Soc. Rev. 52, 4208–4247 (2023).
Puts, G. J., Crouse, P. & Ameduri, B. M. Polytetrafluoroethylene: synthesis and characterization of the original extreme polymer. There was a Chem. Rev. 118.
Baumgartner, R., Stieger, G. K. & McNeill, K. Complete hydrodehalogenation of polyfluorinated and other polyhalogenated benzenes under mild catalytic conditions. Environ. Sci. Technol. 47, 6545–6553 (2013).
Singh, R. K. et al. Rapid removal of poly- and perfluorinated compounds from investigation-derived waste (IDW) in a pilot-scale plasma reactor. It’s in the Environ. Sci. Technol. 53, 11375–11382 (2019).
Singh, K., Kumar, N., Yadav, A. K., Singh, R. & Kumar, K. Per- and polyfluoroalkyl substances (PFAS) as a health hazard: current state of knowledge and strategies in environmental settings across Asia and future perspectives. There is a Chem. Eng. J. 475, 145065 (2023).
N.Yang and others are associated with this work. Solvent-free nonthermal destruction of PFAS chemicals and PFAS in sediment by piezoelectric ball milling. Environ. Sci. Technol. Lett. 10, 198–203 (2023).
Gao, J. et al. The pathways for degradation of photochemical substances are near complete. Nat. Water 1, 381–390, 2023.
Liu, Z. et al. The reaction mechanisms and system efficiency of accelerated degradation of perfluorosulfonates and perfluorocarboxylates. Environ. There is a degree of science mentioned in the area of Technol. 56, 3699–3709 (2022).
Source: Photocatalytic low-temperature defluorination of PFASs
Effects of Gene X and other factors on the development of the zebrafish (An Environ. Health Perspect. 2019)
Gaballah, S, and a few others. Evaluation of the effects of GenX and other factors on the development of the zebrafish. It is referred to as the Environ. Health Perspect. In 2020 there will be 128.
Sunderland, E. M. et al. There are pathways of human exposure to poly- and perfluoroalkyl substances. J. expo. Sci. Environ. Epidemiol 29, 131–147.
Washington, J. W. et al. Nontargeted mass-spectral detection of chloroperfluoropolyether carboxylates in New Jersey soils. Science 368, 1123–1107 was published in 2020.
Resolving Chemical Bonds with Chemists: A Case Study of PTFE-based Photocatalytic Oxidation Catalysts
Only part of the problem can be addressed by any solution that emerges from such developments. It is essential that updated regulation of chemicals and more research into safer alternatives to PFAS that do not harm human health or the environment are done.
Such bonds lie at the heart of per- and polyfluoroalkyl substances (PFAS), a group of compounds, numbering in the millions, that are remarkably water-, heat- and greaseproof. Polytetrafluoroethylene (PTFE, branded as Teflon) was discovered in the 1930s with the purpose of making pans non stick and keeping rain off our jackets. Varieties of cosmetics, fire-retardant foam, kitchen utensils, metal coatings, packaging, textiles and more all contain them.
They are known asforever chemicals because they are hard to break down, and last for a long time in the environment. They are also ‘everywhere’ chemicals, in that they can be found in rivers and on the tops of mountains. Chemicals that are highly toxic have been linked to a variety of problems, from cancer to immune-system suppression.
Both methods combine a catalyst with a relatively easy chemistry that is driven by visible light. The catalyst absorbs light and creates a reaction.
A group of Chemists at Colorado State University use absorbed energy to reduce the C– F bond to carbon–hydrogen, but not in Teflon1. The chemistry department of the University of Science and Technology of China in Hefei uses this energy to break bonds in temperatures as low as 40 C. Both papers show a major step forward.
Next steps include using the ideas to clean up waste water and polluted soils as well as using them in real-world settings. It would be huge benefit if a method could be adapted so that it could run on sunlight.
The European Union ban on persistent pollutants does not apply to medicine and transport. Application to molecule bond production, pharmaceuticals, and medicine
The latest list of banned substances for persistent organic pollutants, which was put out by the Swedish Convention, only included three types of the pollutant.
For the simple reason that these chemicals are too useful, the European proposal doesn’t extend to banning their use in applications like medicine or transport. Molecules need C–F bonds in pharmaceuticals to remain stable for their shelf life.
A group of chemists at Colorado State University in the US used absorbed energy to reduce the C– F bond to Carbon–hydrogen but not in temperatures of 40C or above. The chemistry department of the University of Science and Technology of China in Hefei uses this energy to break bonds in as low as 40C.